Quiz Summary
0 of 33 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 33 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- Thermochemistry 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 33
1. Question
1 point(s)Lead(II) iodide is formed when aqueous lead(II) nitrate is added to a solution
containing iodide ions.
What type of reaction takes place?
Plumbum(II) iodida terbentuk apabila plumbum(II) nitrat akueus dicampur kepada larutan yang
mengandungi ion iodida.
Apakah jenis tindak balas yang berlaku?CorrectIncorrect -
Question 2 of 33
2. Question
1 point(s)Which of the following substance is found in crude oil?
Antara bahan berikut yang manakah terdapat dalam minyak mentah?CorrectIncorrect -
Question 3 of 33
3. Question
1 point(s)2 g of marble is added to 10 cm3 of hydrochloric acid in four different test tubes.
In which test tube is the reaction fastest?2 g marmar ditambah kepada 10 cm3 asid hidroklorik dalam empat tabung uji berlainan.
Dalam tabung uji manakah menghasilkan tindak balas paling cergas?CorrectIncorrect -
Question 4 of 33
4. Question
1 point(s)What are formed when glucose is fermented?
Apakah yang terbentuk dalam penapaian glukosa?CorrectIncorrect -
Question 5 of 33
5. Question
1 point(s)Diagram 6 shows the apparatus set up used to study the reaction of metal oxide and
carbon.
Rajah 6 menunjukkan susunan radas yang digunakan untuk mengkaji tindak balas antara logam oksida
dengan karbon.
Which of the following metal oxide will turn the lime water cloudy?
Antara oksida logam berikut yang manakah mengeruhkan air kapur?CorrectIncorrect -
Question 6 of 33
6. Question
1 point(s)The reaction between solution P and solution Q is exothermic.
A student confirms this statement by mixing equal volumes of the two solutions and
measuring the temperature change.
Which two pieces of apparatus should the student use?
Tindak balas antara larutan P dan Q adalah eksotermik.
Pelajar mengesahkah pernyataan tersebut dengan mencampurkan isipadu yang sama bagi dua
larutan dan mengukur perubahan suhu.
Apakah dua radas yang perlu digunakan?CorrectIncorrect -
Question 7 of 33
7. Question
1 point(s)Diagram 7 shows a set up where hydrogen is passed over a heated metal oxide.
Rajah 7 menunjukkan susunan radas di mana hidrogen dilalirkan atas logam oksida yang panas.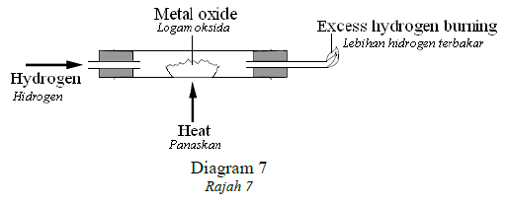
What happens to the hydrogen and metal oxide?
Apa berlaku kepada hidrogen dan oksida logam?CorrectIncorrect -
Question 8 of 33
8. Question
1 point(s)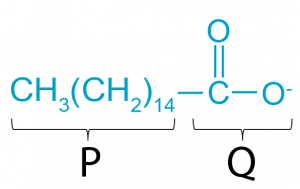
The diagram above shows the structure formula of a palmitate ion. Which of the following statements is true about this ion?
Rajah di atas menunjukkan formula struktur bagi ion palmitat. Antara pernyataan berikut yang manakah benar tentang ion ini?CorrectIncorrect -
Question 9 of 33
9. Question
1 point(s)Diagram 9 shows an experiment in which magnesium oxide powder is added to dilute
hydrochloric acid.
Rajah 9 menunjukkan eksperimen di mana serbuk magnesium oksida ditambah ke dalam asid
hidroklorik cair.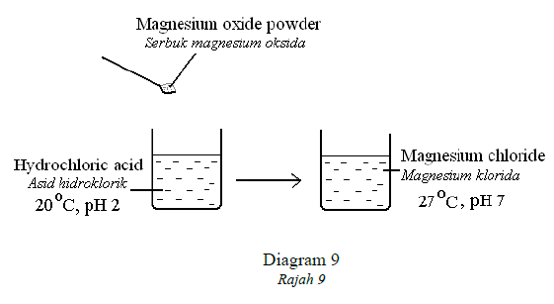
Which reaction takes place in the experiment?
Apakah tindak balas yang terlibat dalam eksperimen tersebut?CorrectIncorrect -
Question 10 of 33
10. Question
1 point(s)When propane is burned in excess oxygen, carbon dioxide and water are formed and
is represented by the equation
Apabila propana dibakar dalam oksigen berlebihan, karbon dioksida dan air dihasilkan dan di wakili
oleh persamaan berikutC3H8 + 5O2 → r CO2 + s H2O
What are the values of r and s that balances the equation?
Apakah nilai r dan s yang mengimbangkan persamaan tersebut?CorrectIncorrect -
Question 11 of 33
11. Question
1 point(s)Diagram12 shows a model of a molecule containing carbon, hydrogen and oxygen.
Rajah 12 menunjukkan model bagi satu molekul yang mengandungi karbon, hidrogen dan oksigen.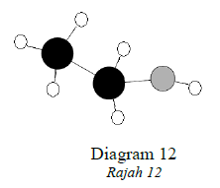
How many atoms of each element are in the molecule?
Berapakah bilangan atom bagi setiap unsur dalam molekul tersebut?CorrectIncorrect -
Question 12 of 33
12. Question
1 point(s)Diagram 13 shows a simple voltaic cell by using different electrode T and N.
Rajah 13 menunjukkan sel kimia ringkas menggunakan elektrod berbeza, T dan N.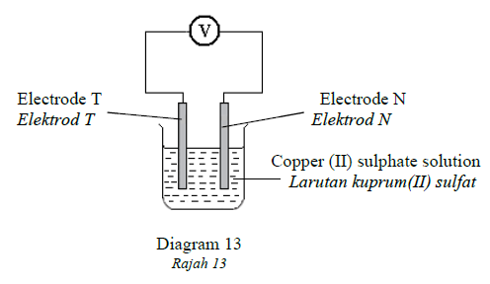
Metal T acts as the positive terminal and the potential difference is 1.9 V.
Which of the following statements is true?
Logam T bertindak sebagai terminal positif dan beza upaya ialah 1.9V .
Antara pernyataan berikut yang manakah benar tentang sel kimia itu?CorrectIncorrect -
Question 13 of 33
13. Question
1 point(s)Diagram 14 shows the pH values of the soil in part F and part G of a palm oil plantation.
Rajah 14 menunjukkan nilai pH bagi tanah di bahagian F dan G bagi suatu ladang kelapa sawit.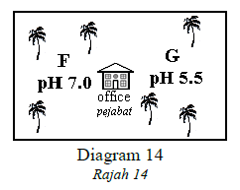
The owner wishes to use lime to neutralise the soil in one part of the plantation
Which part should the lime(calcium oxide) be added, and why?
Pemilik ladang bercadang menggunakan kapur untuk meneutralkan sebahagian daripada ladangnya.
Bahagian manakah yang perlu ditambah kapur dan mengapa?CorrectIncorrect -
Question 14 of 33
14. Question
1 point(s)Diagram 15 shows how ion W in a solution can be identified.
Rajah 15 menunjukkan bagaimana ion W dalam larutan dapat dikenalpasti.
What is ion W?
Apakah ion W?CorrectIncorrect -
Question 15 of 33
15. Question
1 point(s)Polymerization occurs when many small molecules combined to form a long chained
molecule (polymer).
Diagram 16 shows the structure of a small molecule.
Pempolimeran berlaku apabila banyak molekul kecil bergabung membentuk molekul berantai panjang
(polimer).
Rajah 16 menunjukkan struktur suatu molekul kecil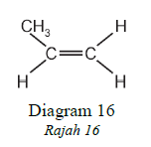
Which of the following represents the structure of a polymer formed when these small
molecules are linked together?
Antara berikut yang manakah mewakili struktur polimer yang terbentuk apabila molekul kecil tersebut
bergabung?CorrectIncorrect -
Question 16 of 33
16. Question
1 point(s)Ethanol reacts with ethanoic acid to form ethyl ethanoate.
Etanol bertindak balas dengan asid etanoik membentuk etil etanoat.C2H5OH + CH3COOH → CH3COOC2H5 + H2O
What is the formula of the ester formed when methanol reacts with butanoic acid,
(C3H7COOH)?
Apakah formula bagi ester yang terbentuk apabila metanol bertindak balas dengan asid butanoik,
(C3H7COOH)?CorrectIncorrect -
Question 17 of 33
17. Question
1 point(s)Diagram 18 shows oxidation process of ethanol.
Rajah 18 menunjukkan proses pengoksidaan etanol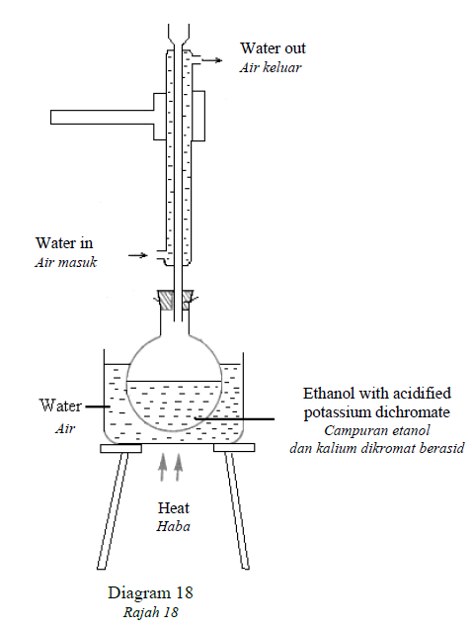
What can be observed after being heated for 5 minutes?
Apakah yang dapat diperhatikan selepas 5 minit dipanaskan?CorrectIncorrect -
Question 18 of 33
18. Question
1 point(s)Diagram 19 shows the apparatus set up used to investigate the rusting of iron.
Rajah 19 menunjukkan susunan radas yang digunakan untuk mengkaji pengaratan besi
In which test-tubes will the iron rust?
Dalam tabung uji manakah besi mengalami pengaratan?CorrectIncorrect -
Question 19 of 33
19. Question
1 point(s)Diagram 20 shows a match. By striking the match, a chemical reaction is initiated.
Rajah 20 menunjukkan sebatang mancis. Apabila mengores mancis, satu tindak balas akan dimulakan.
Which statements about the chemical reaction are correct?
Antara pernyataan berikut yang manakah benar mengenai tindak balas di atas?CorrectIncorrect -
Question 20 of 33
20. Question
1 point(s)Diagram 21 shows apparatus set up used to prepare barium chloride and barium
sulphate. In each experiment, the acid is run into the conical flask until the resulting
liquid has pH7.
Rajah 21 menunjukkan dua susunan radas untuk menghasilkan barium klorida dan barium sulfat.
Dalam setiap eksperimen, asid dititis ke dalam kelalang kon sehingga larutan mencapai pH 7.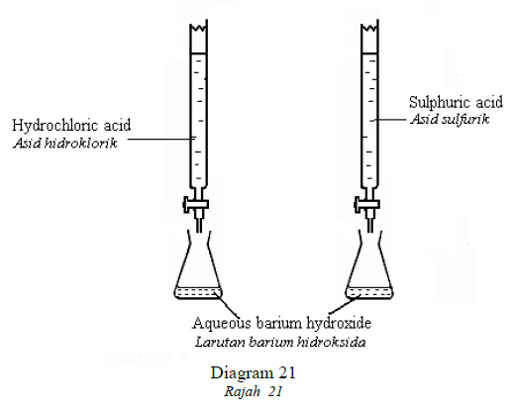
What are the next steps to obtain samples of the solid salts?
Apakah langkah seterusnya untuk mendapatkan garam dalam bentuk pepejal?CorrectIncorrect -
Question 21 of 33
21. Question
1 point(s)For complete combustion, one molecule of an organic compound needs six molecules of oxygen.
What could the formula of this compound be?
Untuk pembakaran lengkap, satu molekul sebatian organik memerlukan enam molekul oksigen.
Apakah formula bagi sebatian tersebut?CorrectIncorrect -
Question 22 of 33
22. Question
1 point(s)Redox reaction between iron(II) ion and manganate(VII) ion is represented by the
equation:
Tindak balas redoks antara ion ferum(II) dan ion manganat(VII) diwakili oleh persamaan:5 Fe2+
+ MnO4
–
+ 8 H+
→ 5 Fe3+
+ Mn2+
+ 4 H2OWhat can be deduced from the equation?
Apakah yang dapat disimpulkan daripada persamaan ini?I The oxidation state of manganese changes from +7 into +2
Nombor pengoksidaan mangan berubah dari +7 kepada +2
II The oxidation state of hydrogen changes from +1 into 0
Nombor pengoksidaan hidrogen berubah dari +1 kepada 0
III The greenish colour of iron(II) ions solution turns to colourless
Warna hijau ion ferum(II) bertukar kepada tidak berwarna
IV Electrons transfer from iron(II) ions to manganate(VII) ions
Pemindahan elektron dari larutan ion ferum(II) kepada ion manganat(VII)CorrectIncorrect -
Question 23 of 33
23. Question
1 point(s)L and M are diatomic elements. L is less reactive than M.
Which of the following elements are L and M?
L dan M adalah unsur-unsur dwiatom.
Antara berikut yang manakah merupakan unsur L dan M?CorrectIncorrect -
Question 24 of 33
24. Question
1 point(s)Heating ammonium salts with sodium hydroxide will produce ammonia gas.
Which of the following ammonium salt can produce the greatest volume of ammonia gas?
Pemanasan garam ammonium dengan natrium hidroksida menghasilkan gas ammonia.
Antara garam ammonium berikut yang manakah akan menghasilkan isipadu gas ammonia tertinggi?CorrectIncorrect -
Question 25 of 33
25. Question
1 point(s)Diagram 23 shows an electrolytic circuit using inert electrodes.
At which electrode is metal deposited?
Rajah 23 menunjukkan satu litar elektrolisis menggunakan elektrod lengai.
Pada elektrod manakah logam terenap?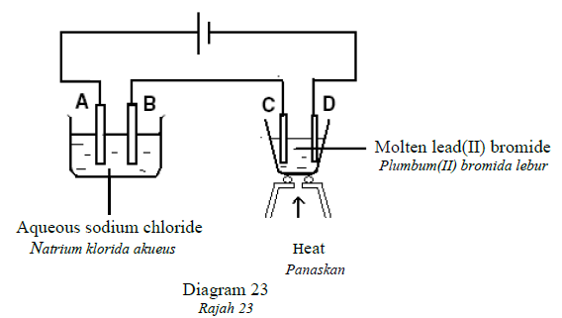 CorrectIncorrect
CorrectIncorrect -
Question 26 of 33
26. Question
1 point(s)Dilute hydrochloric acid was reacted with magnesium ribbon and the volume of
hydrogen gas evolved was measured for the first 80 s.
Asid hidroklorik cair ditindak balaskan dengan pita magnesium dan isipadu gas hidrogen terbebas
diukur bagi 80 saat pertama.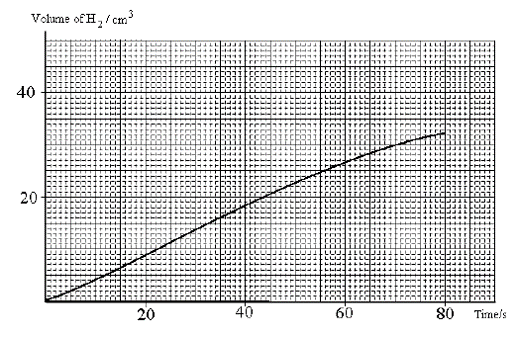
What was the average rate of production of hydrogen?
Apakah kadar purata bagi pembebasan hidrogen?CorrectIncorrect -
Question 27 of 33
27. Question
1 point(s)Diagram 24 shows some reactions of a compound J.
Rajah 24 menunjukkan beberapa tindak balas bagi sebatian J.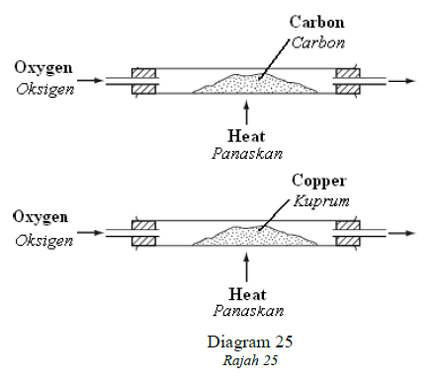
What could compound J be?
Apakah sebatian J?CorrectIncorrect -
Question 28 of 33
28. Question
1 point(s)Calcium carbonate reacts with excess hydrochloric acid at room temperature and is
represented by the equation
Kalsium karbonat bertindak balas dengan asid hidroklorik berlebihan pada suhu bilik dan diwakili
oleh persamaan.
CaCO3 + 2HCl → CaCl2 + H2O + CO2Two experiments were carried out.
Dua eksperimen telah dijalankan.
Experiment I: 10 g of calcium carbonate in large lumps.
Eksperimen I: 10 g ketulan kalsium karbonat
Experiment II: 5 g of calcium carbonate as a fine powder.
Eksperimen II: 5g serbuk kalsium karbonat
Which of the following graph of volume of gas against time is correct?
Antara graf isipadu gas melawan masa berikut yang manakah benar?CorrectIncorrect -
Question 29 of 33
29. Question
1 point(s)Ethene reacts with steam in the presence of a suitable catalyst.
What is the structural formula of the compound formed?
Etena akan bertindak balas dengan stim dalam kehadiran mangkin yang sesuai.
Apakah formula struktur bagi sebatian yang terbentuk?CorrectIncorrect -
Question 30 of 33
30. Question
1 point(s)Powdered carbon and copper are separately heated as shown in Diagram 25.
Serbuk karbon dan kuprum dipanaskan secara berasingan seperti ditunjukkan dalam Rajah 25.
What are the changes in the masses of the powder?
Apakah perubahan jisim bagi kedua-dua serbuk?CorrectIncorrect -
Question 31 of 33
31. Question
1 point(s)Table 5 shows the result of three experiments involving halogen displacement.
Jadual 5 menunjukkan keputusan bagi tiga eksperimen penyesaran halogen.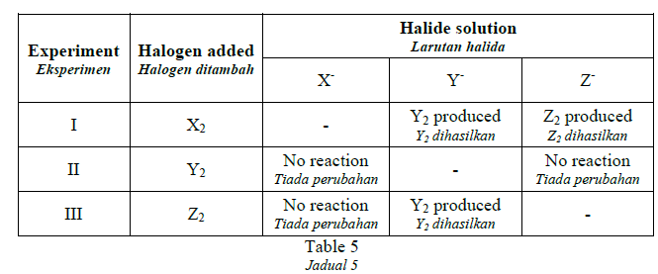
What are halogens X, Y and Z?
Apakah halogen X, Y dan Z?CorrectIncorrect -
Question 32 of 33
32. Question
1 point(s)The thermochemical equation represents the displacement of iron.
Persamaan termokimia mewakili tindak balas penyesaran ferum.Mg(s) + Fe2+
(aq) → Mg2+
(aq) + Fe(s) = -189 kJ mol
1Which of the following statements are true?
Manakah antara berikut benar?
I Magnesium is oxidized
Magnesium dioksidakan
II Reaction is exothermic
Tindak balas adalah eksotermik
III The temperature decreases
Suhu berkurangan
IV The heat released by reacting 0.2 mole of ferum(II) ions is 37.8 kJ
Haba yang dibebaskan semasa 0.2 mol ion ferum(II) bertindak balas ialah 37.8 kJ mol -1CorrectIncorrect -
Question 33 of 33
33. Question
1 point(s)When zinc nitrate is heated strongly, it decomposes according to the equation
Apabila zink nitrat di panaskan dengan kuat, ia akan terurai mengikut persamaan
2 Zn(NO3)2 → 2 ZnO + 4 NO2 + O2Which of the following will be produced when 18.9 g of zinc nitrate is heated?
[Molar mass Zn(NO3)2 = 189 gmol-1, molar mass zinc oxide = 81 gmol-1
and 1 mol
of gas occupies a volume of 24 dm3
at room temperature and pressure]
Antara berikut yang manakah akan terhasil jika 18.9 g zink nitrat dipanaskan?
[Jisim molar: Zn(NO3)2 =189 gmol-1, molar mass ZnO = 81 gmol-1
dan 1 mol gas menempati isipadu
sebanyak 24 dm3
pada suhu dan tekanan bilik]
CorrectIncorrect