Quiz Summary
0 of 46 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 46 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 46
1. Question
Calcium carbonate, CaCO3 is the main component of marble. How many moles of
atom of each element is present in 1 mol of calcium carbonate?
Kalsium karbonat, CaCO3 adalah komponen utama dalam marmar. Berapakah bilangan mol
atom setiap unsur dalam 1 mol kalsium karbonat?CorrectIncorrect -
Question 2 of 46
2. Question
What is the factor that determines the chemical properties of an element?
Apakah faktor yang menentukan sifat kimia sesuatu unsur?CorrectIncorrect -
Question 3 of 46
3. Question
Table 1 shows the proton number for four elements in the Periodic Table.
Jadual 1 menunjukkan nombor proton bagi empat unsur dalam Jadual Berkala.Elements
UnsurProton Number
Nombor protonT 3 U 6 V 11 W 17 Table 1
Jadual 1Which of the following pairs of elements are placed in the same group in the Periodic
Table?
Antara pasangan unsur berikut, yang manakah berada dalam kumpulan sama dalam Jadual
Berkala?CorrectIncorrect -
Question 4 of 46
4. Question
During the formation of ionic bonds, the atoms of elements
Dalam pembentukkan ikatan ion, atom-atom unsurCorrectIncorrect -
Question 5 of 46
5. Question
Which of the following is an example of electrolyte?
Antara berikut, yang manakah adalah contoh elektrolit?CorrectIncorrect -
Question 6 of 46
6. Question
What are the cations present in zinc sulphate solution?
Apakah kation yang hadir dalam larutan zink sulfat?CorrectIncorrect -
Question 7 of 46
7. Question
Why ammonia solution is a weak alkali?
Mengapakah larutan ammonia sejenis alkali lemah ?CorrectIncorrect -
Question 8 of 46
8. Question
Which of the following chemical equations represents the formation of oleum in
Contact Process?
Antara persamaan kimia berikut, manakah mewakili penghasilan oleum dalam Proses Sentuh?CorrectIncorrect -
Question 9 of 46
9. Question
An alloy which is used to make surgical forceps has the following composition:
Sejenis aloi yang digunakan untuk membuat forsep pembedahan mempunyai komposisi
berikut:
Iron
Besi – 74 %
Chromium
Kromium – 18 %
Carbon
Karbon – 8 %
This alloy is strong and does not corrode easily. What is this alloy?
Aloi ini kuat dan tidak mudah terkakis. Apakah aloi ini?CorrectIncorrect -
Question 10 of 46
10. Question
Diagram 1 shows standard representation of chlorine isotopes.
Rajah 1 menunjukkan wakilan piawai isotop-isotop bagi klorin.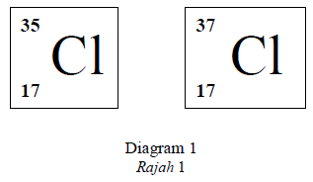
Both isotopes have
Kedua-dua isotop mempunyaiCorrectIncorrect -
Question 11 of 46
11. Question
Which of the following is/are weak acid?
Antara berikut, yang manakah merupakan asid lemah?I HCl
II HNO3
III H2SO4
IV CH3COOHCorrectIncorrect -
Question 12 of 46
12. Question
What should be added to latex so that it stays in liquid form?
Apakah yang perlu ditambahkan kepada lateks bagi mengekalkannya dalam bentuk cecair?CorrectIncorrect -
Question 13 of 46
13. Question
Diagram 2 shows the mixture of liquid Z and water in a test tube.
Rajah 2 menunjukkan campuran cecair Z dan air dalam tabung uji.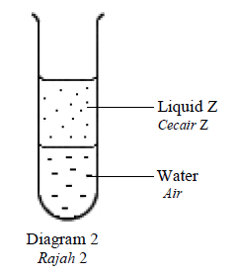
Which of the following is liquid Z?
Antara berikut, yang manakah merupakan cecair Z?CorrectIncorrect -
Question 14 of 46
14. Question
Which of the following chemical reactions is a redox reaction?
Antara tindak balas kimia berikut, yang manakah merupakan satu tindak balas redoks?CorrectIncorrect -
Question 15 of 46
15. Question
Diagram 3 shows the rusting process of an iron nail when paired with metal X.
Rajah 3 menunjukkan proses pengaratan paku besi bila dipasangkan dengan logam X.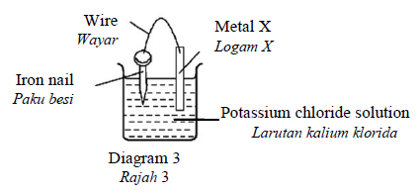
The rate of rusting is the highest when X is
Kadar pengaratan adalah paling tinggi apabila X adalahCorrectIncorrect -
Question 16 of 46
16. Question
Which of the following pairs of additive in detergent and its function is correct?
Antara pasangan berikut, manakah bahan tambah detergen dan fungsinya yang benar?CorrectIncorrect -
Question 17 of 46
17. Question
Diagram 4 shows the application of chemical reactions in daily life.
Rajah 4 menunjukkan aplikasi tindak balas kimia dalam kehidupan seharian.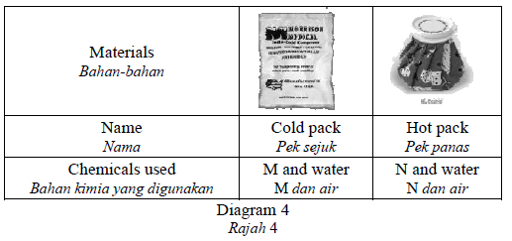
What is M and N?
Apakah M dan N?CorrectIncorrect -
Question 18 of 46
18. Question
A student has a whooping cough.
He went to a clinic and the doctor prescribed streptomycin.
Seorang pelajar mengalami batuk kokol.
Dia pergi ke klinik dan doktor memberinya streptomisin.What type of medicine is streptomycin?
Apakah jenis ubat streptomisin?CorrectIncorrect -
Question 19 of 46
19. Question
The following shows a standard representation of potassium atom, K.
Berikut adalah wakilan piawai bagi atom kalium K.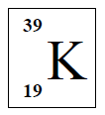
Which is the correct electron arrangement for potassium?
Susunan elektron manakah yang betul bagi kalium?CorrectIncorrect -
Question 20 of 46
20. Question
Table 2 shows the melting and boiling points of substances V, W, X and Y.
Jadual 2 menunjukkan takat lebur dan takat didih bahan V, W, X dan Y.Substances
Bahan-bahanMelting point (oC)
Takat lebur (
oC)Boiling point (oC)
Takat didih (
oC)V – 23 7 W 64 298 X – 256 – 256 Y 12 135 Table 2
Jadual 2Which of the following substances is in liquid form at room temperature?
Antara bahan berikut, manakah merupakan cecair pada suhu bilikCorrectIncorrect -
Question 21 of 46
21. Question
Why the reactivity of Group 1 elements increases when going down the group?
Mengapa kereaktifan unsur Kumpulan 1 meningkat apabila menuruni kumpulan?CorrectIncorrect -
Question 22 of 46
22. Question
Table 3 shows the electron arrangement of atoms J and L.
Jadual 3 menunjukkan susunan elektron bagi atom J dan L.Atom Electron arrangement
Susunan elektronJ 2.8.2 L 2.8.7 Which of the following is true for the compound formed when J reacts with L?
Antara berikut manakah benar bagi sebatian yang terbentuk apabila J bertindak balas dengan
L?CorrectIncorrect -
Question 23 of 46
23. Question
Diagram 5 shows a chemical cell using magnesium and copper as the electrodes.
Rajah 5 menunjukkan sel kimia menggunakan magnesium dan kuprum sebagai elektrod.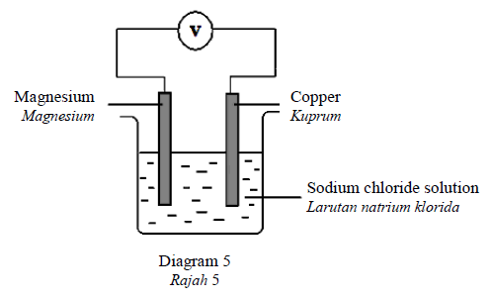
Which of the following half equations represents the reaction at the copper electrode?
Antara persamaan setengah berikut, yang manakah mewakili tindak balas yang berlaku di
elektrod kuprum?CorrectIncorrect -
Question 24 of 46
24. Question
The pH of 0.1 mol dm-3
hydrochloric acid, HCl and 0.1 mol dm-3
sulphuric acid,
H2SO4 are not the same because
pH asid hidroklorik, HCl 0.1 mol dm-3 dan asid sulfurik, H2SO4 0.1 mol dm-3 berbeza keranaCorrectIncorrect -
Question 25 of 46
25. Question
Diagram 6 shows the reaction of lead(II) nitrate with solution X.
Rajah 6 menunjukkan tindak balas antara plumbum(II) nitrat dengan larutan X.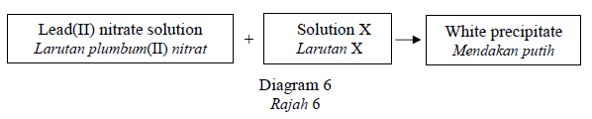
What is the solution X?
Apakah larutan X?CorrectIncorrect -
Question 26 of 46
26. Question
Pure metals are ductile and malleable. This is because
Logam tulen adalah mulur dan boleh ditempa. Ini adalah keranaCorrectIncorrect -
Question 27 of 46
27. Question
Diagram 7 shows a graph of the volume of gas produced against time for the reaction
between zinc granules and hydrochloric acid.
Rajah 7 menunjukkan graf isipadu gas yang dihasilkan melawan masa bagi tindak balas
antara ketulan zink dan asid hidroklorik.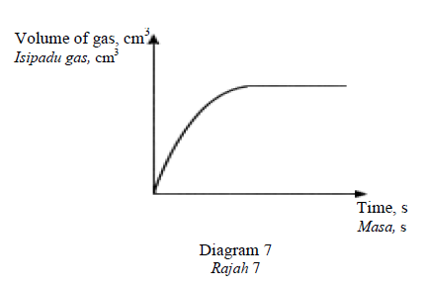
The gradient of the graph decreases with time because
Kecerunan graf berkurang dengan masa keranaCorrectIncorrect -
Question 28 of 46
28. Question
Magnesium reacts with acid to produce hydrogen gas, H2.
Which solution would give the highest initial rate of reaction?
Magnesium bertindak balas dengan asid untuk menghasilkan gas hidrogen, H2.
Larutan manakah akan memberikan kadar awal tindak balas yang tertinggi?CorrectIncorrect -
Question 29 of 46
29. Question
Both ethane and ethene
Kedua-dua etana dan etenaCorrectIncorrect -
Question 30 of 46
30. Question
The following equation represents a chemical reaction of ethanol.
Persamaan berikut mewakili satu tindak balas kimia bagi etanol.What can be used to identify substance P?
Apakah yang boleh digunakan untuk mengenal pasti bahan P?CorrectIncorrect -
Question 31 of 46
31. Question
When 50 cm3
of 1.0 mol dm-3
nitric acid is mixed with 50 cm3
of 1.0 mol dm-3
sodiumhydroxide solution the temperature increases by 6.0 oC .
What is the temperature change if the experiment is repeated using 50 cm3
of2.0 mol dm-3 nitric acid with 50 cm3
of 1.0 mol dm-3 sodium hydroxide?
Apabila 50 cm3
asid nitrik 1.0 mol dm-3 dicampurkan dengan 50 cm3
natrium hidroksida
1.0 mol dm-3, suhu bertambah sebanyak 6.0 oC.
Berapakah perubahan suhu jika eksperimen diulangi menggunakan 50 cm3asid nitrik
2.0 mol dm-3 dengan 50 cm3
natrium hidroksida 1.0 mol dm-3?
CorrectIncorrect -
Question 32 of 46
32. Question
A student carries out an experiment to determine the heat of combustion of propanol.
Which of the following information does he need in order to calculate the heat of
combustion?
Seorang pelajar menjalankan eksperimen untuk menentukan haba pembakaran bagi propanol.I Mass of water
Jisim air
II Volume of propanol
Isipadu propanol
III Initial temperature of propanol
Bacaan suhu awal propanol
IV Highest temperature of water
Bacaan suhu tertinggi air
Antara maklumat berikut, yang manakah diperlukan untuk menentukan haba pembakaran
propanol?CorrectIncorrect -
Question 33 of 46
33. Question
Table 5 shows four elements and their proton numbers.
Jadual 5 menunjukkan empat unsur dan bilangan proton.Element
UnsurProton number
Nombor protonP 8 Q 11 R 17 S 16 Table 5
Jadual 5Given the proton number of flourine is 9, which of the following elements has similar
chemical properties to it?
Jika nombor proton bagi florin ialah 9, manakah antara unsur berikut mempunyai sifat kimia
yang sama dengannya?CorrectIncorrect -
Question 34 of 46
34. Question
Table 6 shows the electron arrangement and nucleon number for atoms E and G.
Jadual 6 menunjukkan susunan elektron dan nombor nukleon bagi atom E dan G.Atom E Atom G Electron arrangement
Susunan elektron2.8.3 2.8.7 Nucleon number
Nombor nukleon27 35 Table 6
Jadual 6Based on Table 6, what is the relative molecular mass for compound formed when E
reacts with G?
Berdasarkan Jadual 6, apakah jisim molekul relatif bagi sebatian yang terbentuk apabila atom
E bertindak balas dengan atom G?CorrectIncorrect -
Question 35 of 46
35. Question
An element J forms compound JCl3 with chlorine and JSO4 with sulphate ion.
Which of the following is true for this element?
Unsur J membentuk sebatian JCl3 dengan klorin dan JSO4 dengan ion sulfat.
Antara berikut yang manakah benar tentang unsur ini?CorrectIncorrect -
Question 36 of 46
36. Question
R reacts with S to form ionic compound with a formula of R2S3.
Which of the following electron arrangements are true for R and S atoms?
R bertindak balas dengan S membentuk sebatian ion dengan formula R2S3.
Antara susunan elektron berikut yang manakah benar bagi atom R dan S?CorrectIncorrect -
Question 37 of 46
37. Question
Table 7 shows the potential differences for three simple cells.
Jadual 7 menunjukkan beza upaya bagi tiga sel ringkas.Pair of metals
Pasangan logamPotential difference (V)
Beza upaya (V)Negative terminal
Kutub negatifK and copper
K dan kuprum0.4 K K and copper
K dan kuprum1.3 L M and copper
M dan kuprum0.6 Cu Table 7
Jadual 7Based on the potential values given, what is the arrangement of all metals K, L, M and
copper in ascending order of electropositivity?
Berdasarkan kepada beza upaya yang diberikan, manakah susunan kesemua logam K, L, M
dan kuprum mengikut urutan keelektropositifan menaik?CorrectIncorrect -
Question 38 of 46
38. Question
Diagram 8 shows the set-up of apparatus for the titration of sodium hydroxide solution
with hydrochloric acid.
Rajah 8 menunjukkan susunan radas bagi pentitratan larutan natrium hidroksida dengan asid
hidroklorik.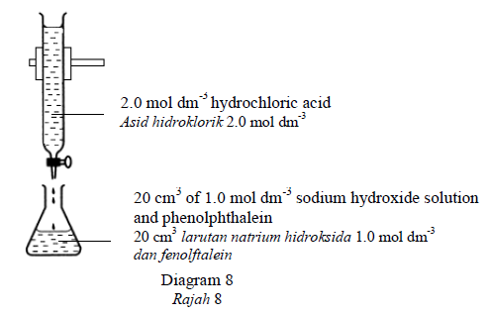
What is the total volume of the mixture in the conical flask at end point?
Berapakah jumlah isipadu campuran larutan di dalam kelalang kon pada takat akhir
pentitratan?CorrectIncorrect -
Question 39 of 46
39. Question
The following equation represents the reaction between magnesium oxide and nitric
acid.
Persamaan berikut mewakili tindak balas antara magnesium oksida dengan asid nitrik.MgO + 2HNO3 → Mg(NO3)2 + H2O
Excess magnesium oxide is reacted with 50 cm3
of 2.0 mol dm-3 nitric acid.
What is the maximum mass of magnesium nitrate salt formed?
[Relative atomic mass: N = 14, O = 16, Mg = 24]
Magnesium oksida yang berlebihan bertindak balas dengan 50 cm3asid nitrik 2.0 mol dm-3
.Apakah jisim maksimum garam magnesium nitrat yang terbentuk?
[Jisim atom relatif: N = 14, O = 16, Mg = 24]CorrectIncorrect -
Question 40 of 46
40. Question
The following chemical equation represents the complete combustion of propane.
Persamaan kimia berikut mewakili pembakaran lengkap propana.C3H8 + 5O2 → 3CO2 + 4H2O
What is the volume of oxygen gas used if 5.5 g of propane is completely burnt in air?
[Relative atomic mass: H = 1, C = 12, O = 16;
Molar volume of gas = 24 dm3mol-1 at room conditions]
Berapakah isipadu gas oksigen yang digunakan jika 5.5 g propana terbakar lengkap dalam
udara?
[Jisim atom relatif: H = 1, C = 12, O = 16; Isipadu molar gas = 24 dm3mol-1 pada keadaan
bilik]
CorrectIncorrect -
Question 41 of 46
41. Question
Diagram 10 shows the set-up of apparatus of an electrolysis process.
Rajah 10 menunjukkan susunan radas bagi satu proses elektrolisis.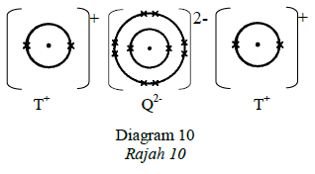
Which of the following electrolytes produces oxygen gas at electrode U?
Antara elektrolit berikut, yang manakah menghasilkan gas oksigen di elektrod U?CorrectIncorrect -
Question 42 of 46
42. Question
The following statements describe the particles of a substance at room temperature.
Pernyataan-pernyataan berikut menerangkan tentang zarah-zarah suatu bahan pada suhu
bilik.
• The particles are far apart from each other.
Zarah-zarah adalah berjauhan antara satu sama lain.
• Forces of attraction between particles are weak.
Daya tarikan antara zarah adalah lemah.
• The particles have high kinetic energy and move randomly.
Zarah-zarah mempunyai tenaga kinetik yang tinggi dan bergerak rawak..
Which of the following substance match the criteria?
Antara bahan berikut, yang manakah memenuhi kriteria di atas?CorrectIncorrect -
Question 43 of 46
43. Question
Diagram 11 shows the preparation of standard solution of potassium hydroxide, KOH
by dissolving 5.6 g of potassium hydroxide in distilled water and making the volume
up to 100 cm3
.Rajah 11 menunjukkan penyediaan larutan piawai kalium hidroksida, KOH dengan
melarutkan 5.6 g kalium hidroksida di dalam air suling dan menjadikan isipadu sehingga 100
cm3.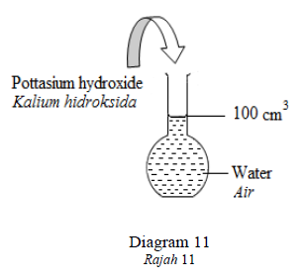
What is the volume of the standard solution prepared above that should be used if a
student wants to prepare 50 cm3of 0.5 mol dm-3 potassium hydroxide solution?
[Relative formula mass: KOH = 56]
Berapakah isipadu larutan piawai yang disediakan di atas perlu digunakan jika seorang
pelajar ingin menyediakan 50 cm3larutan kalium hidroksida 0.5 mol dm-3?
[Jisim formula relatif: KOH = 56]
CorrectIncorrect -
Question 44 of 46
44. Question
Table 9 shows the experiments carried out to study the rate of reaction between zinc
carbonate and nitric acid.
Jadual 9 menunjukkan eksperimen yang dijalankan bagi mengkaji tindak balas antara zink
karbonat dengan asid nitrik.
Which of the following graph represents the two experiments?
Antara graf berikut yang manakah mewakili kedua-dua eksperimen itu?CorrectIncorrect -
Question 45 of 46
45. Question
The following equations represent the displacement reaction of metals.
Persamaan-persamaan berikut mewakili tindak balas penyesaran beberapa logam.
Reaction I : E + G2+→ E2+ + G
Tindak balas I
Reaction II : L + E2+→ L2+ + E
Tindak balas II
Which of the following statement is true?
Antara pernyataan berikut yang manakah benar?CorrectIncorrect -
Question 46 of 46
46. Question
Diagram 12 shows an energy level diagram for the reaction between acid and alkali.
Rajah 12 menunjukkan rajah aras tenaga bagi tindak balas antara asid dan alkali.
Calculate the amount of heat released when 50 cm3 of 2 mol dm-3 ethanoic acid reacts with 50 cm3 of 2 mol dm-3 potassium hydroxide solution.
Hitung jumlah haba yang dibebaskan apabila 50 cm3 asid etanoik 2 mol dm-3 bertindak balas dengan 50 cm3 larutan kalium hidroksida 2 mol dm-3.CorrectIncorrect